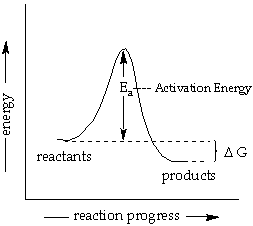
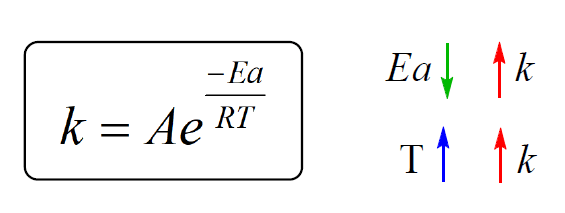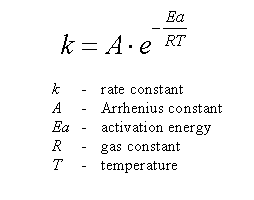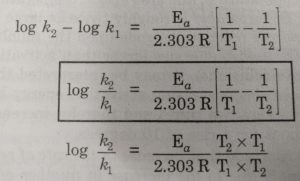Calculate Activation Energy for a Reaction of Which Rate Constant Becomes Four Times When Temperature Changes from 30 °C to 50 °C - Chemistry | Shaalaa.com

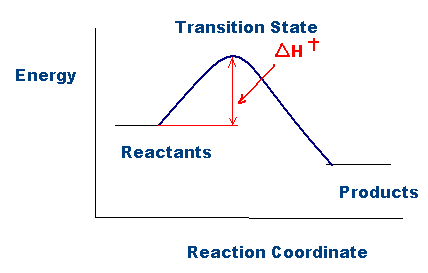
Title: Lesson 6 Activation Energy Learning Objectives: – Understand the term activation energy – Calculate activation energy from experimental data. - ppt download
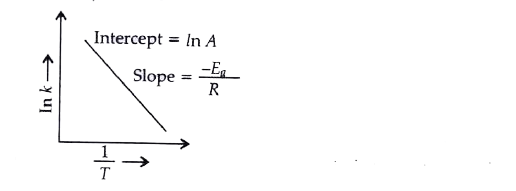
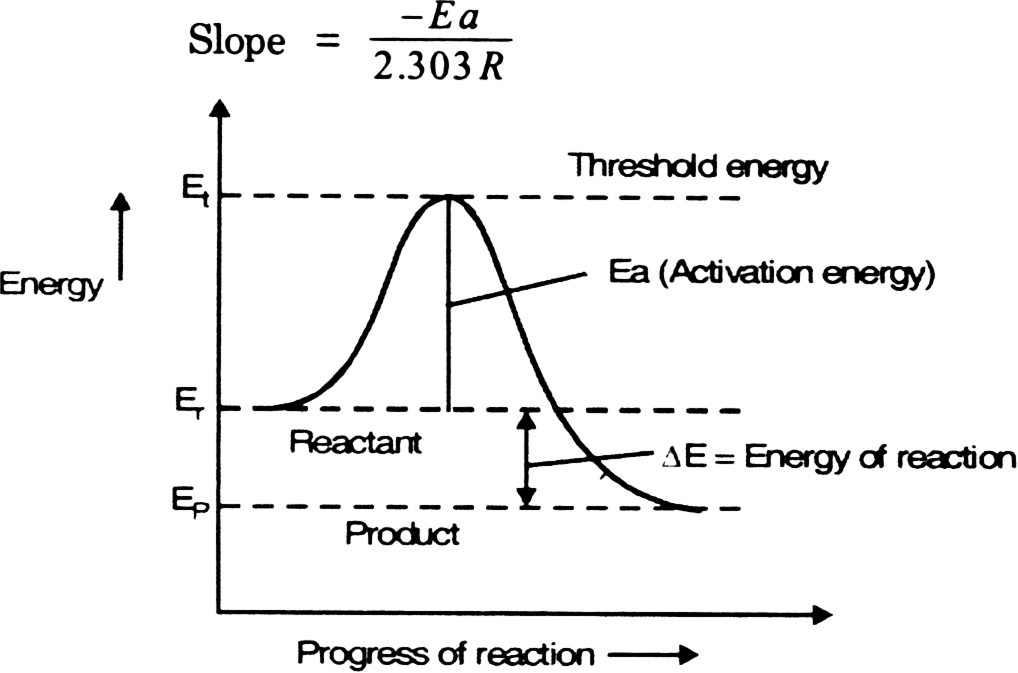
Draw a graph which is used to calculate the activation energy of a reaction. Give the appropriate expressions used to calculate the activation energy graphically.

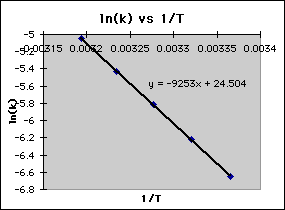
Calculate the activation energy, Ea, in kilojoules per mole for a reaction at 65.0 C that has a rate constant of 0.288 s?1 and a frequency factor of 7.56 1011 s?1. | Homework.Study.com
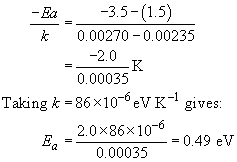
Engineering: The challenge of temperature: 4.3.3 Getting at the activation energy - OpenLearn - Open University

Difference Between Activation Energy and Threshold Energy | Compare the Difference Between Similar Terms


:max_bytes(150000):strip_icc()/thermometer--28-degrees-celsius--heat-day-533592578-596394475f9b583f180f036f.jpg)